CHO Stable Cell Line Development
Precision Antibody now offers stable mammalian cell line development for high titer, scalable manufacture of monoclonal antibodies and recombinant proteins. Under license from Life Technologies, cell lines are generated using a parental line derived from Chinese hamster ovary (CHO) DG44 cells, which are optimized for growth in suspension in chemically defined media, and for recombinant protein expression. These cell lines are derived from GMP-banked DG44 cells for maximal traceability. Commercial-use licenses to use these cell lines are available for biotechnology, pharmaceutical, vaccine production, veterinary, human diagnostic, and other companies interested in using our cell lines to produce proteins for sale. Licenses are priced competitively, and include access to a complete documentation package with detailed lineage information, as well as testing data. The documentation packages greatly simplify regulatory submissions for the resulting proteins and can be included with New Drug Applications for FDA filings.
Cell Line Development Services
We offer an array of services to simplify cell line development. From vector design to accession cell banking, our industry experienced scientists and project managers are able to provide support of biotechnology products destined for regulatory approval.
Parental Cell Lines
For biomanufacturing, the objective is to create a scalable expression system with superior yields of a quality product. Our CHO DG44 cells are derived from a cGMP master cell bank licensed from Life Technologies. The cells have been adapted to CD DG44 Medium for serum free suspension growth. This medium is made from animal origin free and chemically defined components. It contains no proteins, hydrolysates, or components of unknown composition.
Vector Design and Construction
Expression levels can be systematically enhanced by creating an optimal vector, which contains the desired genetic elements. DG44 System has minimal licensing requirements and royalties, and is regulatory-friendly. Sequences of monoclonal antibodies and proteins originally discovered using platforms such as hybridoma and phage display can be transferred to our mammalian expression vectors for transient production and cell line generation.
Transient Protein Expression
Transient transfection of mammalian cells has become increasingly important to rapidly produce milligram quantities of a recombinant protein for characterization and preclinical studies. We offer transient production of mAbs and recombinant proteins in CHO-S suspension culture up to multi-liter scale in less than two weeks. Purification of the transiently expressed protein is available based on your requirements.
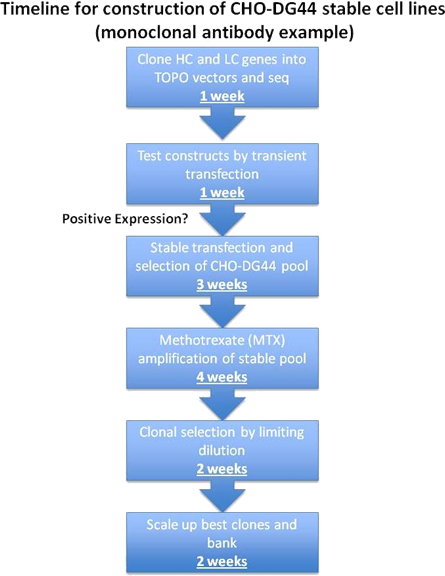
Stable Cell Line Development
Stable CHO DG44 cell lines are created using a suite of optimized media, reagents, and protocols that have been optimized to shorten time lines with improved efficiencies. DG44 cells are used to generate the best performing suspension-adapted stable pool. Further increases in specific productivity are gained by genetic amplification and the highest expressing clones are isolated by limiting dilution.
Cell Line Stability Evaluation
Cell line stability is a critical factor for process scale up. For large-scale commercial manufacturing, cell lines routinely grow for over 25 generations to achieve high-density culture in the 10,000-20,000 liter production bioreactor. Studies to determine cell line stability are performed up to 50 generations. Best performing cell line will be propagated in small suspension culture and scaled to the 5-L bioreactor at interim time points to evaluate cell growth and product yield and determine long-term cell line stability.
Accession Cell Banking (ACB) for Individual Clonal Cell Lines
Following characterization of clonal cell lines, the best performing cell line from each lineage will be banked to generate Accession Cell Banks (ACBs) which will be tested for sterility and mycoplasma. ACBs may be subsequently used in further process optimization and scale-up studies. As product development nears regulatory filing, a Master Cell Bank (MCB) and Working Cell Bank (WCBs) may be generated from ACB vials.
To contact our staff for your next cell line development project:
Call us at 410-884-4100, email orders@precisionantibody.com, or try our online quote request form. We provide free consultation and an exact price quote for all stable cell line development projects.
